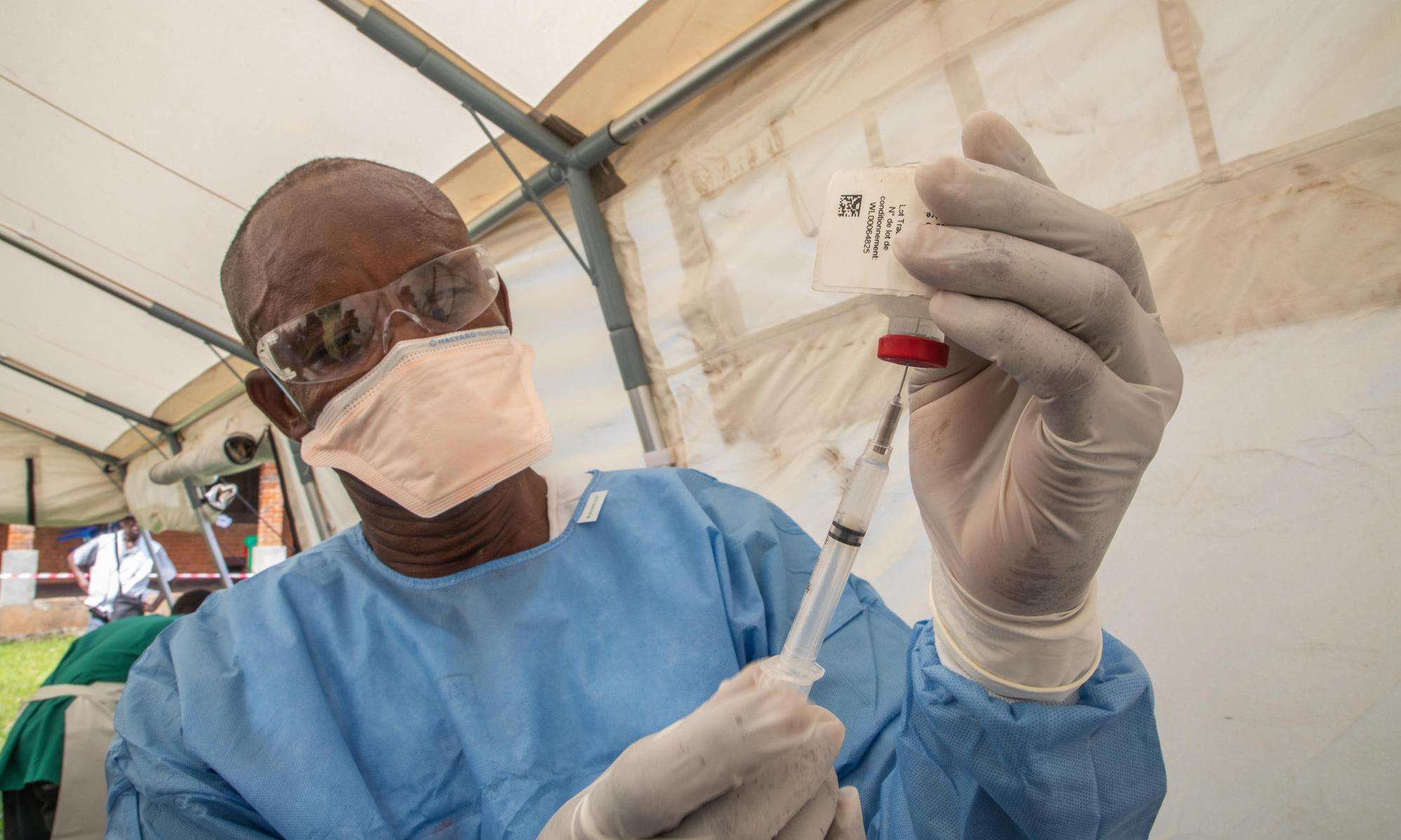
Stockpile is a step in the right direction, but more transparency and research needed to fully meet the needs of people at risk of contracting Ebola
NEW YORK, JANUARY 12, 2021—The international medical humanitarian organization Doctors Without Borders/Médecins Sans Frontières (MSF) is among a group of international partners that announced today the creation of a global stockpile of the Ervebo Ebola vaccine. This stockpile will help countries and treatment providers like MSF ensure people have access to Ebola vaccines.
The Ervebo vaccine, which is manufactured by Merck and is effective in preventing the most common Ebola strain, Zaire, was licensed in 2019 by the US Food and Drug Administration and the European Medicines Agency before being prequalified by the World Health Organization (WHO) and approved by eight African countries. Between 2018 and 2020, the vaccine was used during the tenth and largest Ebola outbreak in the history of the Democratic Republic of Congo (DRC).
The stockpile will be housed in Switzerland and managed by an International Coordinating Group (ICG), which includes MSF among other organizations. WHO’s Strategic Advisory Group of Experts on Immunization working group recommended that an emergency global stockpile of this vaccine consist of 500,000 doses. However, there are currently only 6,890 doses available because of supply bottlenecks—and it may take up to three years for the stockpile to contain the target number of doses.
Dr. Natalie Roberts, MSF Foundation’s program manager for epidemics, said the following of today’s announcement:
"The creation of an Ebola vaccine stockpile under the ICG is a positive step.
“Vaccination is one of the most effective ways to respond to Ebola outbreaks. The deployment of a vaccine was among the key innovations in the response to recent outbreaks in DRC. An Ebola vaccine stockpile can increase transparency in the management of existing global stocks and the timely deployment of the vaccine where it's most needed, addressing the concerns that MSF raised during the tenth Ebola outbreak in DRC.
“But efforts toward progress in Ebola vaccination must now continue.
“For example, the limited size of the stockpile shouldn’t restrict further study regarding the most appropriate vaccination strategies to better respond to Ebola epidemics in the future. Equally, research should continue to make this vaccine more adapted to the contexts in which Ebola epidemics occur, in particular, advancing toward a more stable product not requiring a complex cold chain for storage and transport. In consideration of the limited number of doses currently available in the stockpile, it will be important that ICG partners are able to resolve any supply bottlenecks and quickly secure additional doses should another large-scale outbreak be declared. Lastly, the creation of this stockpile should not prevent the ongoing development of complementary vaccines and treatments that would further contribute to reducing the incidence and mortality related to this disease.”