Georgia 2016 © Daro Sulakauri/MSF
Georgia
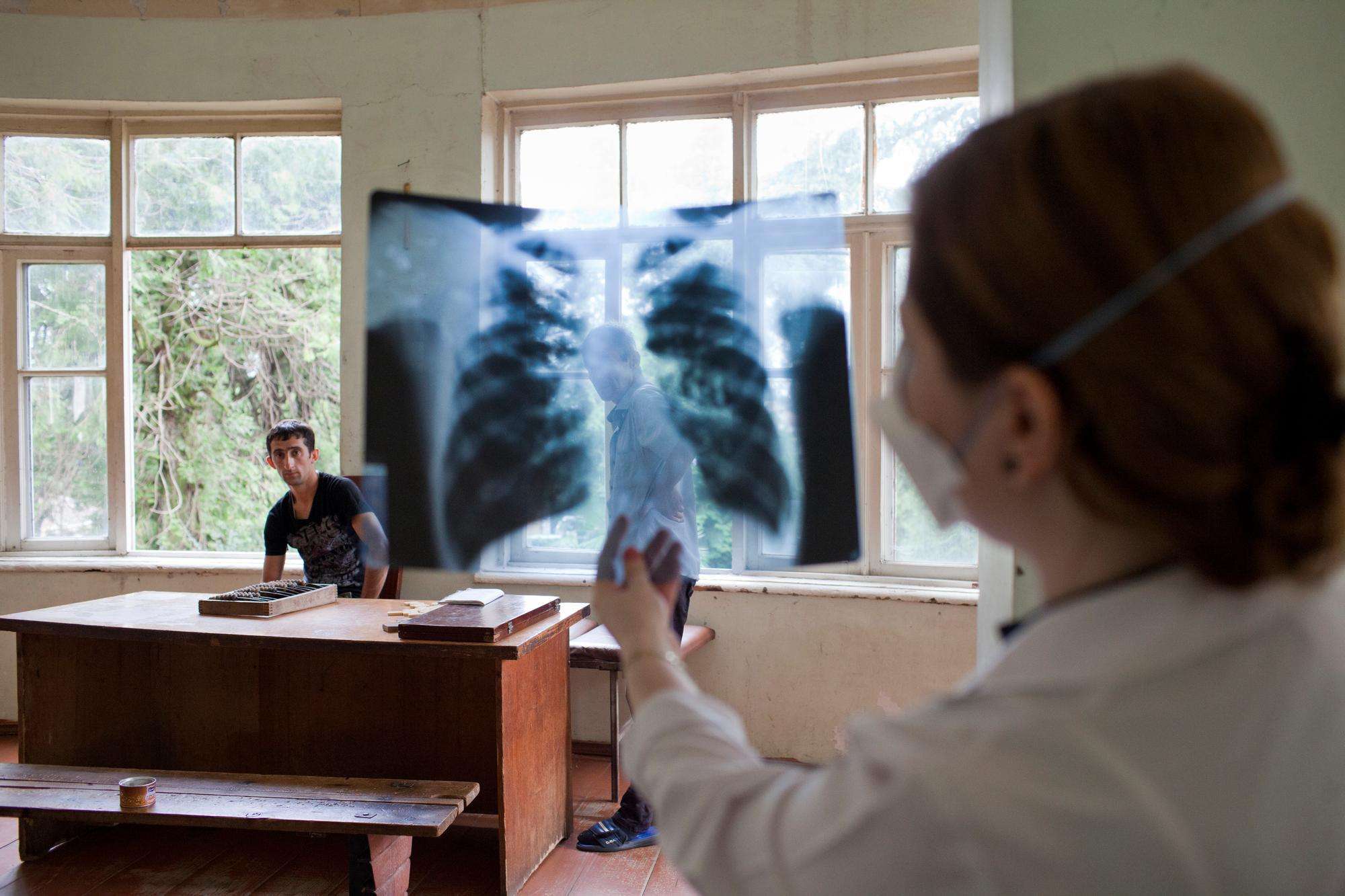
Continuing efforts to combat multidrug-resistant tuberculosis
End of medical activities
MSF ceased working in Georgia in 2020 with the last follow up of the trial’s patients.
Our Work in Georgia
After 25 years in Georgia, Doctors Without Borders/Médecins Sans Frontières (MSF) activities, which have mainly focused on the treatment of multidrug-resistant tuberculosis (MDR-TB), are nearing completion.
What is happening in Georgia?
Georgia is one of the 30 countries with the highest rates of MDR-TB. MSF first supported TB activities in Abkhazia and South Ossetia regions between 1993 and 1994, and drug-resistant TB (DR-TB) care in Abkhazia between 2001 and 2014. Learn how you can best help in Georgia and other countries.
How we're helping in Georgia
In 2014, we began supporting the use of bedaquiline in Georgia through the compassionate use mechanism, whereby patients with life-threatening conditions gain access to investigational drugs. The following year, Georgia became one of 17 participating countries in the endTB observational study of bedaquiline and delamanid to find shorter, more tolerable, injection-free treatments for MDR-TB. A total of 297 patients were enrolled in this study. In 2017, the first patient was enrolled in the endTB clinical trial—a randomized trial that followed the endTB study in the search for better MDR-TB treatment.
Today, Georgia is one of only four MDR-TB high-burden countries to have implemented all-oral regimens for more than 95 percent of MDR-TB patients. Please donate to support our work in Georgia and other countries around the world now.
MSF began working in Georgia in 1993 to deliver health care to people affected by internal displacement and conflict. Activities included surgery, vaccinations, and supplying drugs to health facilities. Over the following years, we provided assistance to Chechen refugees in Pankisi Valley, running medical and surgical programs as well as donating drugs. From 2000, we expanded our activities to include general health care for vulnerable people and treatment for visceral leishmaniasis.
When the last patients in the endTB trial complete their follow-up in 2020, MSF programs in Georgia will close.



